The Food and Drug Administration (FDA) granted the emergency use for quick COVID-19 test from Abbott Laboratories. The test was designed for pharmacists services, critical care centers, and small clinics without advanced testing labs. The award took place on March 27.
According to the Illinois-based group, the test is able to produce positive outcomes in as little as five minutes, and opposing impacts in about 13 minutes.
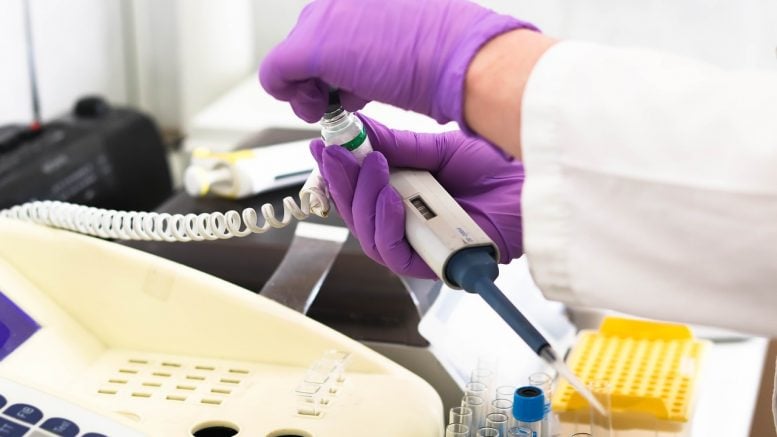
The test works on Abbott’s ID NOW equipment, which is about the size of the microwave oven and is currently employed by many areas to immediately test for flu, strep, and respiratory syncytial virus (RSV) while sufferers require therapy. For coronavirus, health providers would have to order an extra $40 cartridge, about the size of a replacement ink cartridge for printers, mainly founded to pick up hereditary signatures of the SARS-CoV-2 infection.
The Basis Of Test Implementation
The majority of insurers have promised to pay off the price of the test, which is about the same amount as the ID NOW test for flu, without further charges to the sufferers who restrain.
The Abbott test, like the gold-colored regulation COVID-19 test produced by the U.S. administration, applies medicines to destroy the virus’ outer shell, so it drops out its hereditary material, in the form of RNA.
Then synthetic reactions increase genetic material characteristics, so individual molecular examinations in the test resolution can pick up even small quantities of the disease. That’s where the rapid test deviates from the others in use nowadays. With conventional technology, that increase process claims regularly cycling the temperature of the synthetic reactions from low to average to large and back down again.
The ID NOW equipment is able to complete the genetic development at a consistent temperature, which means it can spit out decisions more swiftly.
Thus why aren’t all coronavirus tests run using the more agile technology? One reason has to do with volume; standard genetic tests can prepare hundreds or even thousands of searches a day.
The ID NOW technology can only run about four units an hour. And while standard tests may take longer to provide outcomes, the investigators don’t require to spend as much time explaining the test itself so it can start examining people sooner.
The fast test “catches quite a bit of optimization and refining,” states John Frels, the vice president of analysis and improvement at Abbott Diagnostics. It means that fast tests need more upfront improvement time and takes more long-drawn to work.
Device Characteristics
For practitioner services, critical care centers, and tinier hospitals without advanced lab amenities on-site, the automatic test could be a game-changer. Physicians could operate the test while the sufferers are waiting and give results in a few seconds rather than several days.
Frels states that if a sufferer has high levels of illness in their unit—taken from the back of the throat and nose—the test’s radar is able to score the threshold for a specific diagnosis in a few five minutes.
The compact device also can indicate a sea difference in the way health care will be presented in the upcoming years. Simultaneously, the fast testing platform was initially used for a few respiratory conditions, regarding it in an impasse pandemic setting could scale up testing rapidly and suppress a rising tide of delivery.
Frels also asserts that Abbott is ramping up the stock of COVID-19 test cartridges and plans to spread enough to provide 50,000 tests per day by next week.



Be the first to comment on "COVID-19 Test From Abbott"