Yesterday the U.S. Food and Drug Administration (FDA) accepted Tepezza (teprotumumab-trow) for the processing of adults with a thyroid eye disorder, a rare condition where the muscles and fatty tissues behind the eye become swollen, causing the eyes to be strained forward and bulge outwards (proptosis). Yesterdays’s endorsement signifies the first medication approved for the therapy of thyroid eye syndrome.
“The approval signals an essential milestone for the therapy of thyroid eye disorder. Currently, there are insignificant processing options for this possibly debilitating illness.
This therapy has the potential to alter the course of the condition, possibly sparing patients from needing multiple invasive operations by providing an option, nonsurgical therapy choice,” stated Wiley Chambers, M.D., deputy administrator of the Division of Transplant and Ophthalmology Products in the FDA’s Center for Drug Evaluation and Research.
Spezza Basics
“Furthermore, thyroid eye syndrome is a rare disorder that impacts a tiny percentage of the community, and for a variety of reasons, therapies for rare illnesses are often unavailable. This consent represents significant progress in support of effective treatments for rare illnesses, such as thyroid eye condition.”
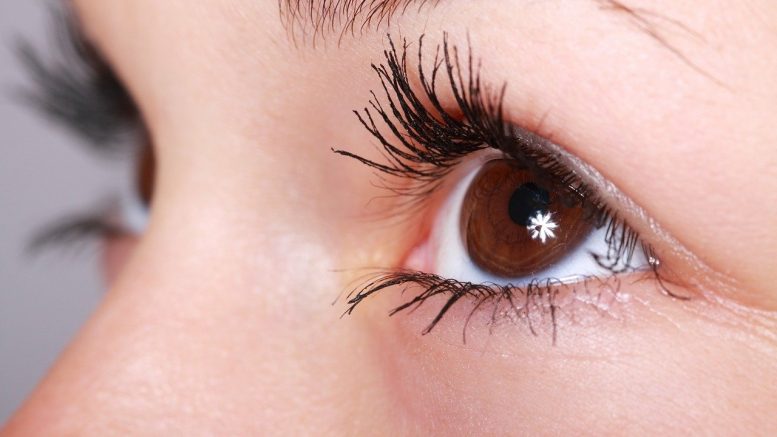
Thyroid eye syndrome is associated with the outward bulging of the eye that can cause a type of symptoms such as eye pain, double vision, light feeling, or difficulty closing the eye. This condition impacts a comparatively small number of Americans, with more women than men interested.
Although this condition afflicts approximately a few individuals, thyroid eye disease can be damaging. For example, the troubling ocular symptoms can lead to the growing inability of people with thyroid eye illness to perform critical daily activities, such as driving or operating.
Spezza was established based on the results of two studies (Study 1 and 2) consisting of a total of 170 sufferers with existing thyroid eye syndrome who were randomized to either get Tepezza or a placebo. Of the sufferers who were given Tepezza, 71% in Study 1 and 83% in Study 2 showed a more meaningful than the 2-millimeter decline in proptosis (eye protrusion) as compared to 20% and 10% of subjects who received placebo, severally.
The most current opposing reactions observed in patients treated with Tepezza are muscle spasm, nausea, alopecia (hair loss), diarrhea, fatigue, hyperglycemia (high blood sugar), hearing loss, dry skin, dysgeusia (altered sense of taste) and headache.
Beginning Therapy
Spezza should not be used if pregnant, and women of child-bearing potential should have their pregnancy status checked before beginning therapy and should be taught on pregnancy prevention during treatment and for six months following the last dose of Tepezza.
The FDA awarded this application Priority Review, in addition to Fast Track and Breakthrough Therapy Designation. Additionally, Tepezza obtained Orphan Drug designation, which provides considerations to assist and support the development of drugs for rare illnesses or conditions.
The configuration of this product was also in part sponsored by the FDA Orphan Products Grants Program, which provides grants for clinical studies on the security and efficacy of products for use in rare diseases or conditions.
To Sum Up
The FDA admitted the approval of Tepezza to Horizon Therapeutics Ireland DAC.
The FDA, an agency within the U.S. Department of Health and Human Services, preserves the public health by ensuring the safety, effectiveness, and security of human and veterinary medicines, vaccines and other biological outcomes for human use, and medical devices. The agency also is accountable for the safety and security of our nation’s food supply, cosmetics, dietary complements, products that give off electronic radiation, and for regulating tobacco goods.




Be the first to comment on "FDA Enables Treatment For Thyroid Eye Disease"